Evaluation of Safety: Monotherapy
The safety profile of Myrbetriq has been evaluated in adult patients with overactive bladder (OAB) in three 12-week trials and a 1-year safety study.1
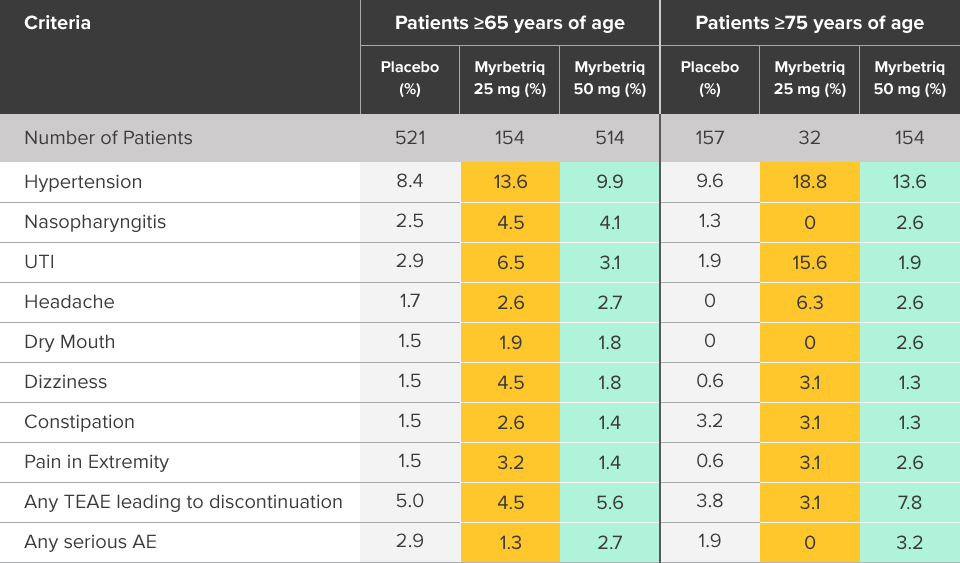
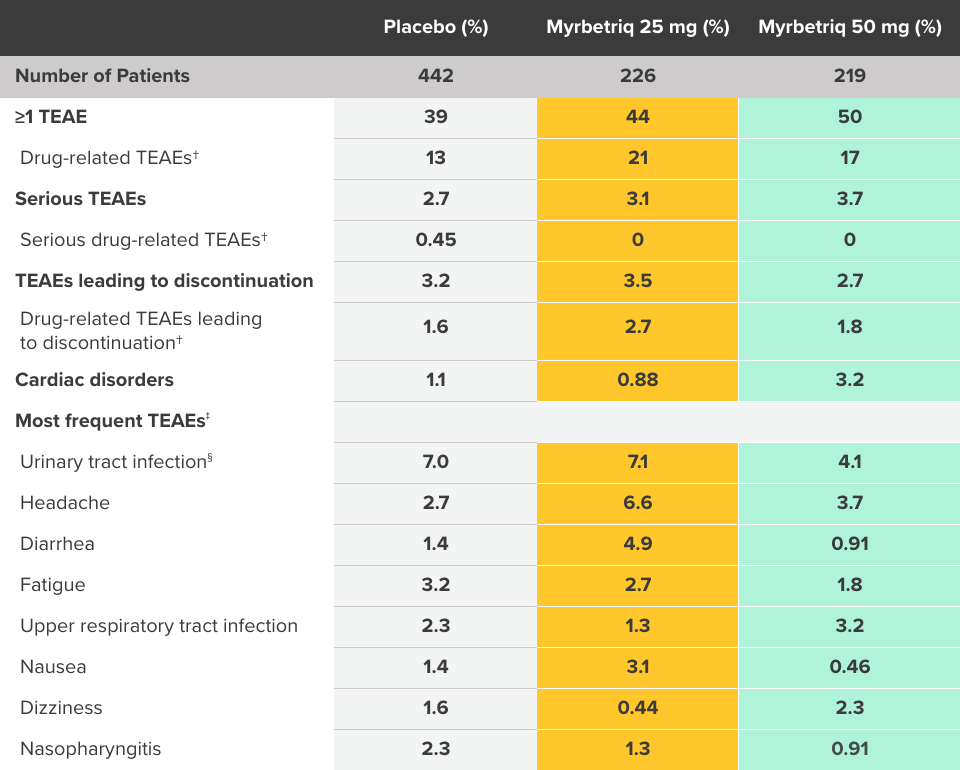
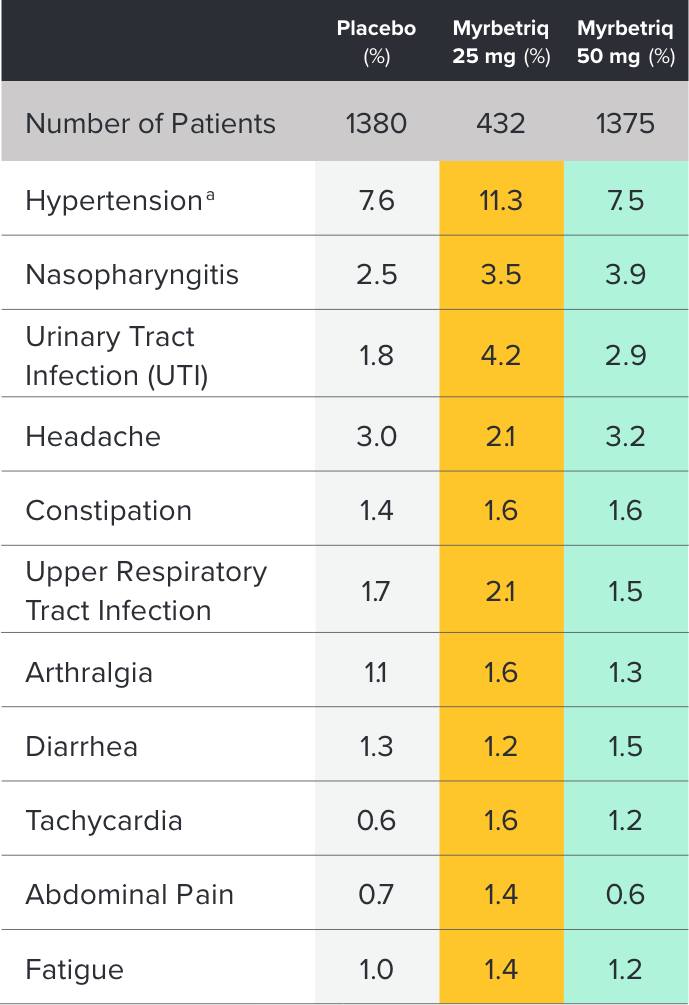
Safety and tolerability studied in three 12-week phase III trials1
Percent of patients with adverse reactions derived from all adverse events, exceeding placebo rate and reported by ≥1% of patients treated with Myrbetriq 25 mg or 50 mg once daily in Studies 1, 2, and 31
aIncludes reports of blood pressure (BP) above the normal range and BP increased from baseline, occurring predominantly in subjects with baseline hypertension.
Myrbetriq was evaluated in three 12‑week, double‑blind, randomized, placebo‑controlled, parallel‑group, multicenter clinical trials in patients with OAB.1
- The most frequent adverse events (0.2%) leading to discontinuation in Studies 1, 2, and 3 for the 25 mg or 50 mg dose were nausea, headache, hypertension, diarrhea, constipation, dizziness, and tachycardia
- Atrial fibrillation (0.2%) and prostate cancer (0.1%) were reported as serious adverse events by >1 patient and at a rate greater than placebo
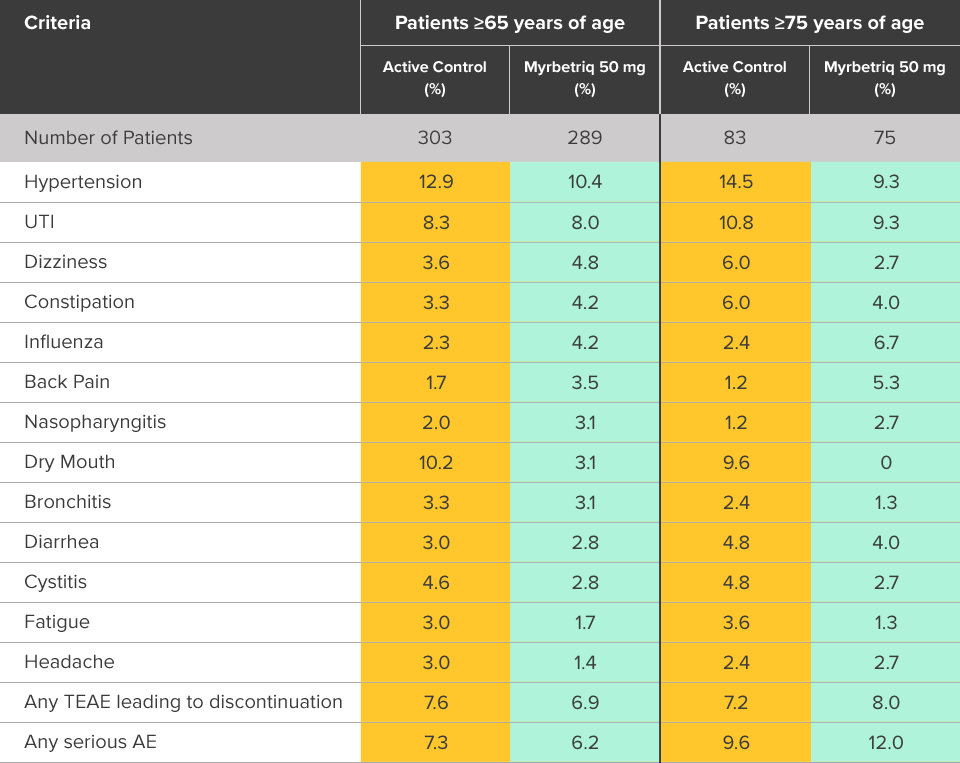
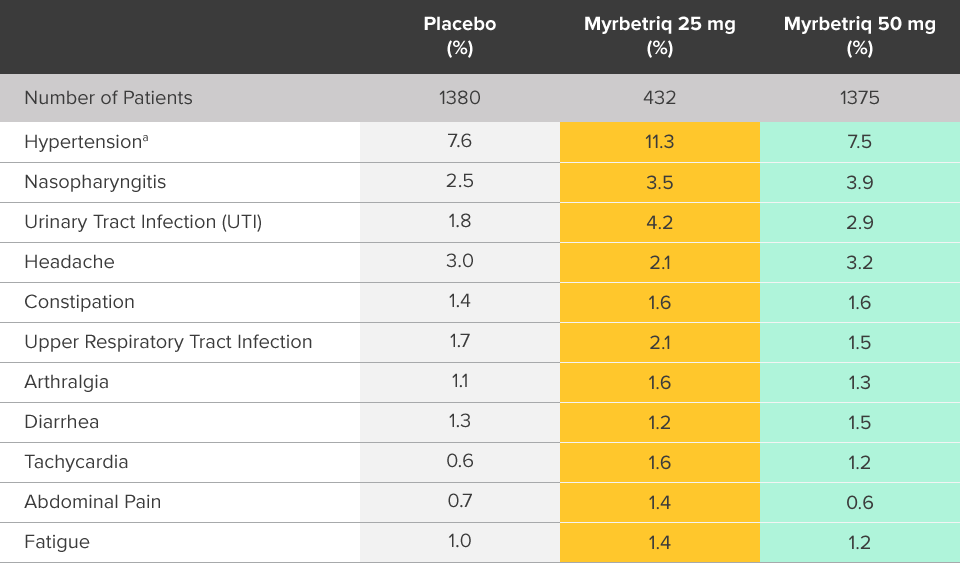
Safety evaluated in a 1-year, randomized, fixed-dose, double‑blind, active-controlled study1
Percent of patients with adverse reactions derived from all adverse events, reported by >2% of patients treated with Myrbetriq 50 mg once daily in the 1 year safety study1
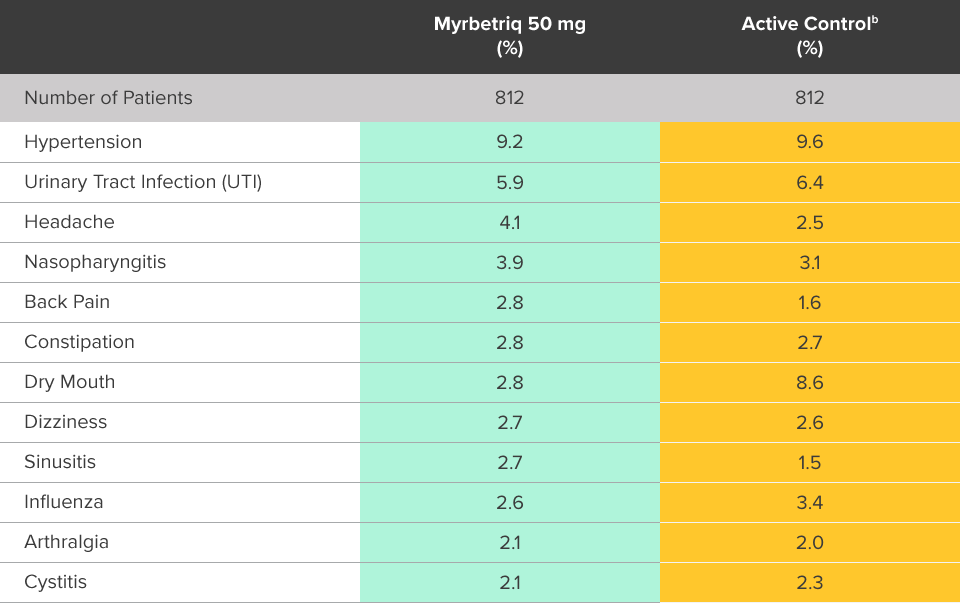
bThe active control in this study was tolterodine extended-release (ER) 4 mg.2
Myrbetriq was evaluated in a 1-year, randomized, fixed‑dose, double‑blind, active‑controlled study1
- Adverse reactions leading to discontinuation in patients treated with Myrbetriq 50 mg (reported by >2 patients and >active control) included constipation (0.9%), headache (0.6%), dizziness (0.5%), hypertension (0.5%), dry eyes (0.4%), nausea (0.4%), vision blurred (0.4%), and urinary tract infection (0.4%)
- Serious adverse events (reported by ≥2 patients and >active control) included cerebrovascular accident (0.4%) and osteoarthritis (0.2%)
- Serum ALT/AST increased from baseline by greater than 10 fold in 2 patients (0.3%) taking Myrbetriq 50 mg; these markers subsequently returned to baseline while both patients continued Myrbetriq
- Serious adverse events of neoplasm were reported by 0.1%, 1.3%, and 0.5% of patients treated with Myrbetriq 50 mg, 100 mg, and active control once daily, respectively. Neoplasms reported by 2 patients treated with Myrbetriq 100 mg included breast cancer, lung neoplasm malignant, and prostate cancer. A causal relationship between mirabegron and these reported neoplasms has not been established
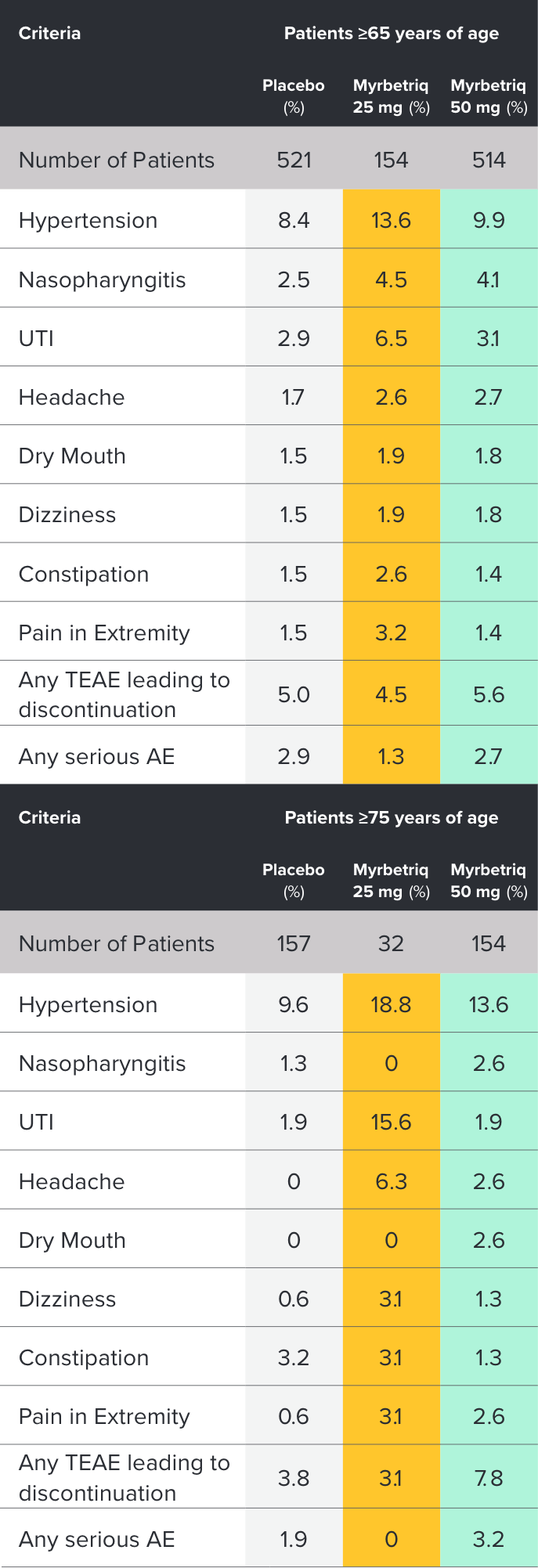
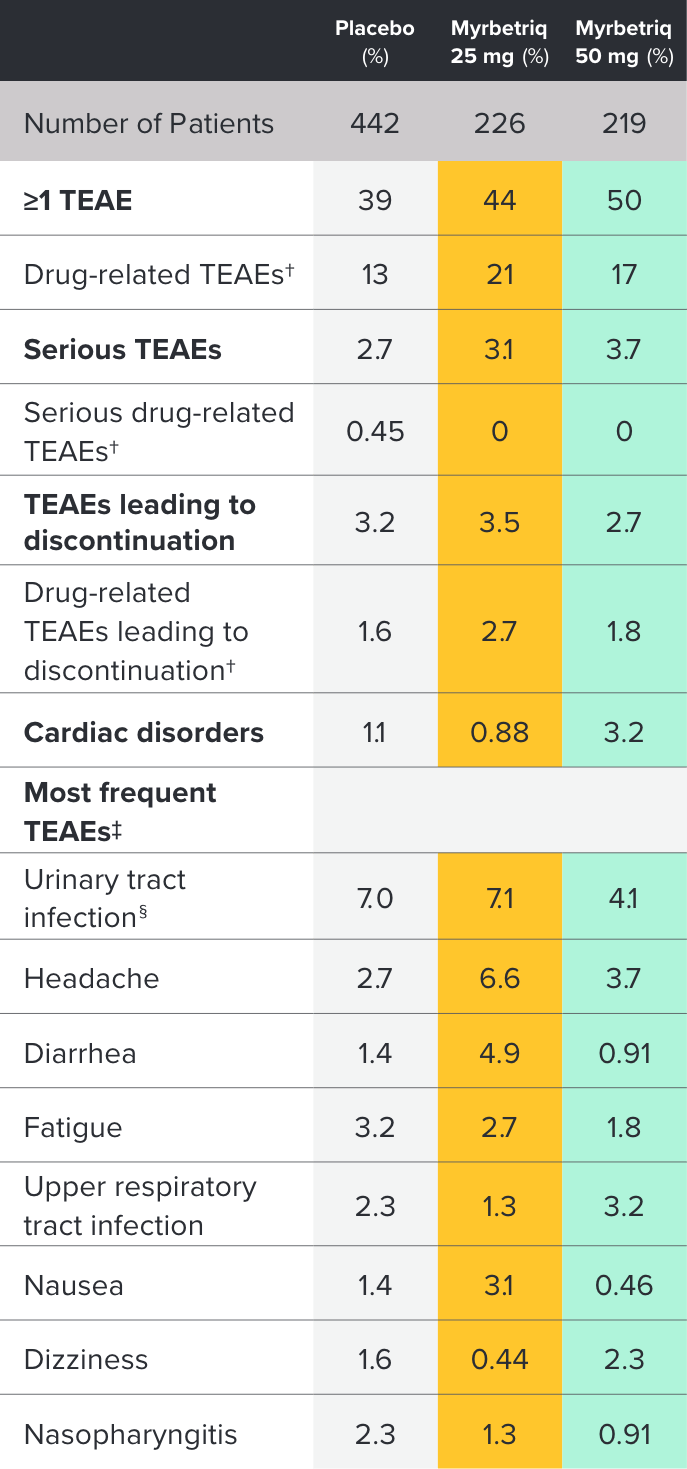
Safety and tolerability studied in three 12-week phase III trials1
Percent of patients with adverse reactions derived from all adverse events, exceeding placebo rate and reported by ≥1% of patients treated with Myrbetriq 25 mg or 50 mg once daily in Studies 1, 2, and 31
aIncludes reports of blood pressure (BP) above the normal range and BP increased from baseline, occurring predominantly in subjects with baseline hypertension.
Myrbetriq was evaluated in three 12‑week, double‑blind, randomized, placebo‑controlled, parallel‑group, multicenter clinical trials in patients with OAB.1
- The most frequent adverse events (0.2%) leading to discontinuation in Studies 1, 2, and 3 for the 25 mg or 50 mg dose were nausea, headache, hypertension, diarrhea, constipation, dizziness, and tachycardia
- Atrial fibrillation (0.2%) and prostate cancer (0.1%) were reported as serious adverse events by >1 patient and at a rate greater than placebo
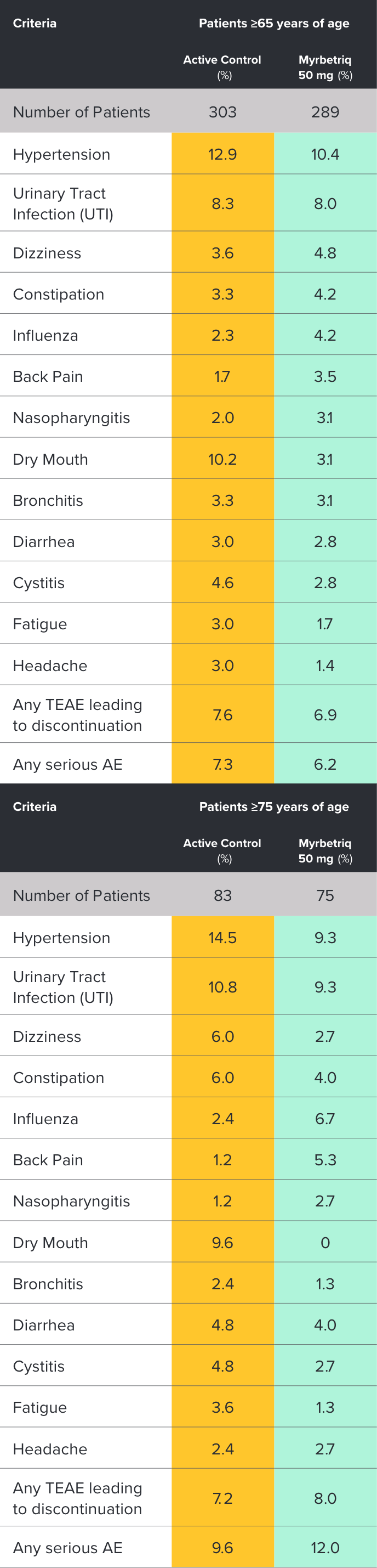
Safety evaluated in a 1‑year, randomized, fixed-dose, double‑blind, active-controlled study1
Percent of patients with adverse reactions derived from all adverse events, reported by >2% of patients treated with Myrbetriq 50 mg once daily in the 1 year safety study1
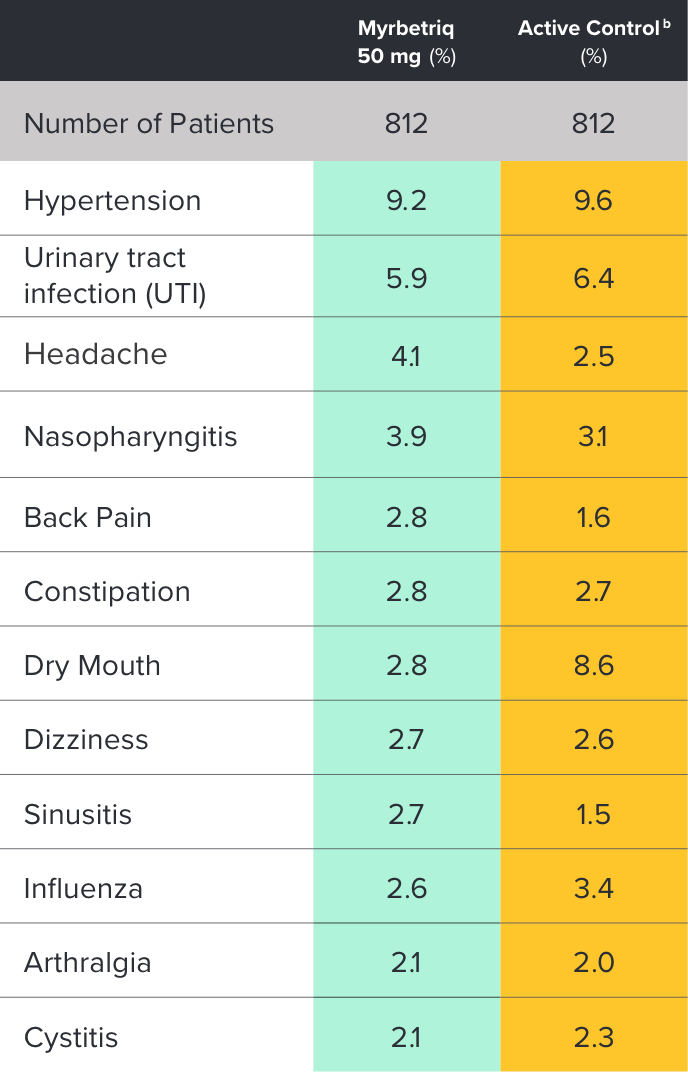
bThe active control in this study was tolterodine extended-release (ER) 4 mg.2
Myrbetriq was evaluated in a 1‑year, randomized, fixed‑dose, double‑blind, active‑controlled study1
- Adverse reactions leading to discontinuation in patients treated with Myrbetriq 50 mg (reported by >2 patients and >active control) included constipation (0.9%), headache (0.6%), dizziness (0.5%), hypertension (0.5%), dry eyes (0.4%), nausea (0.4%), vision blurred (0.4%), and urinary tract infection (0.4%)
- Serious adverse events (reported by ≥2 patients and >active control) included cerebrovascular accident (0.4%) and osteoarthritis (0.2%)
- Serum ALT/AST increased from baseline by greater than 10 fold in 2 patients (0.3%) taking Myrbetriq 50 mg; these markers subsequently returned to baseline while both patients continued Myrbetriq
- Serious adverse events of neoplasm were reported by 0.1%, 1.3%, and 0.5% of patients treated with Myrbetriq 50 mg, 100 mg, and active control once daily, respectively. Neoplasms reported by 2 patients treated with Myrbetriq 100 mg included breast cancer, lung neoplasm malignant, and prostate cancer. A causal relationship between mirabegron and these reported neoplasms has not been established