Your elderly patients ≥65 years can see meaningful improvement in daily OAB symptoms of urinary incontinence and frequency with Myrbetriq.3†
†Myrbetriq was evaluated in a pre-specified subgroup analysis of patients ≥65 years of age from three Phase III pivotal clinical trials. Myrbetriq was also studied in the PILLAR trial–a prospective, double-blind, randomized, placebo-controlled, parallel-group, multicenter Phase IV study of community-dwelling patients ≥65 years of age with wet OAB symptoms for ≥3 months.

See clinical studies, efficacy results, and safety, including a subset analysis of elderly patients
The PILLAR study is the first prospective study to examine the efficacy, safety, and tolerability of Myrbetriq in adults ≥65 years of age with wet OAB symptoms3
Study Design: PILLAR, the First Prospective Study of Myrbetriq in Adults ≥65 Years of Age
Efficacy demonstrated in a double-blind, randomized, placebo-controlled, parallel-group, multicenter Phase IV study3
Efficacy Endpoints
- Co-primary Endpoint: Change from baseline to end of treatment (EoT) in the mean number of urinary incontinence episodes per 24 hours
- Co-primary Endpoint: Change from baseline to EoT in the mean number of micturitions per 24 hours
- Secondary Endpoint: Change from baseline to EoT in mean volume voided per micturition
Entry Criteria
- Male and female community-dwelling patients ≥65 years of age
- Wet OAB symptoms (defined as urgency, urinary frequency, and urinary incontinence) for ≥3 months
- ≥8 micturition episodes per 24 hours
- ≥3 urgency episodes
- ≥1 urinary incontinence episode
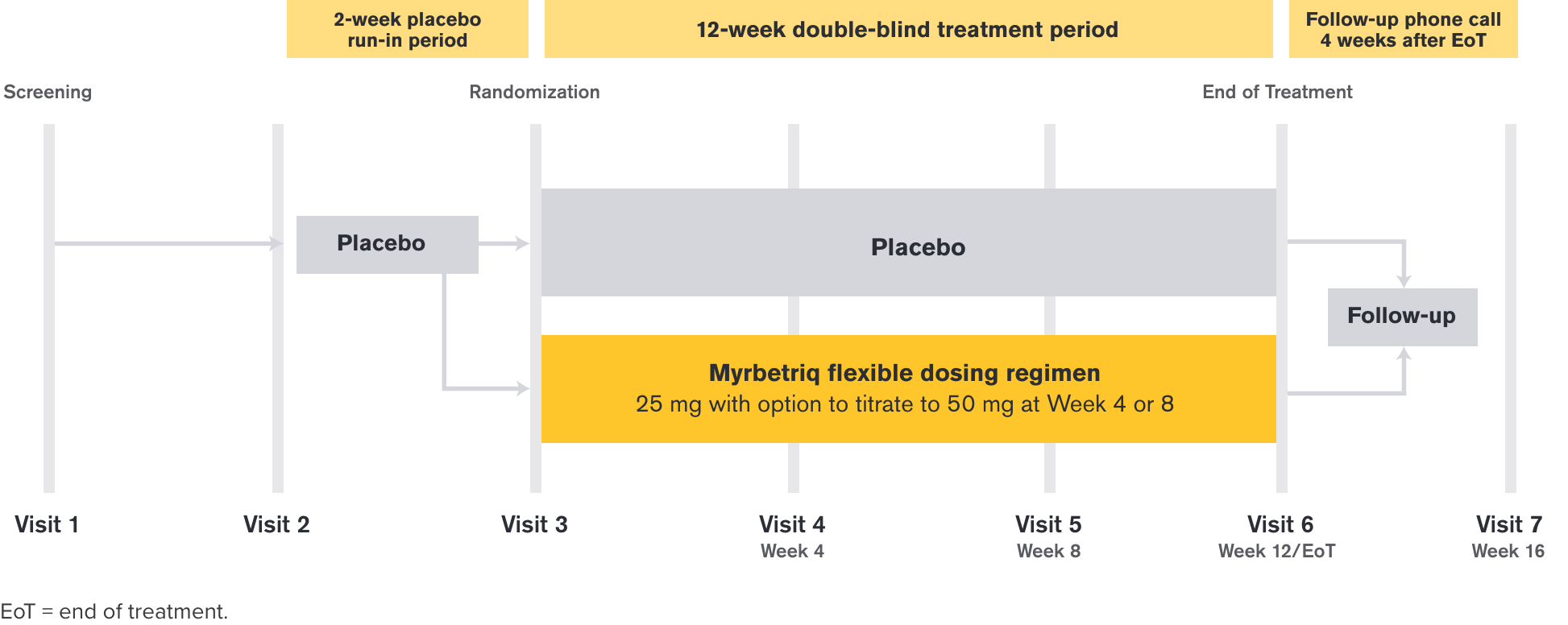
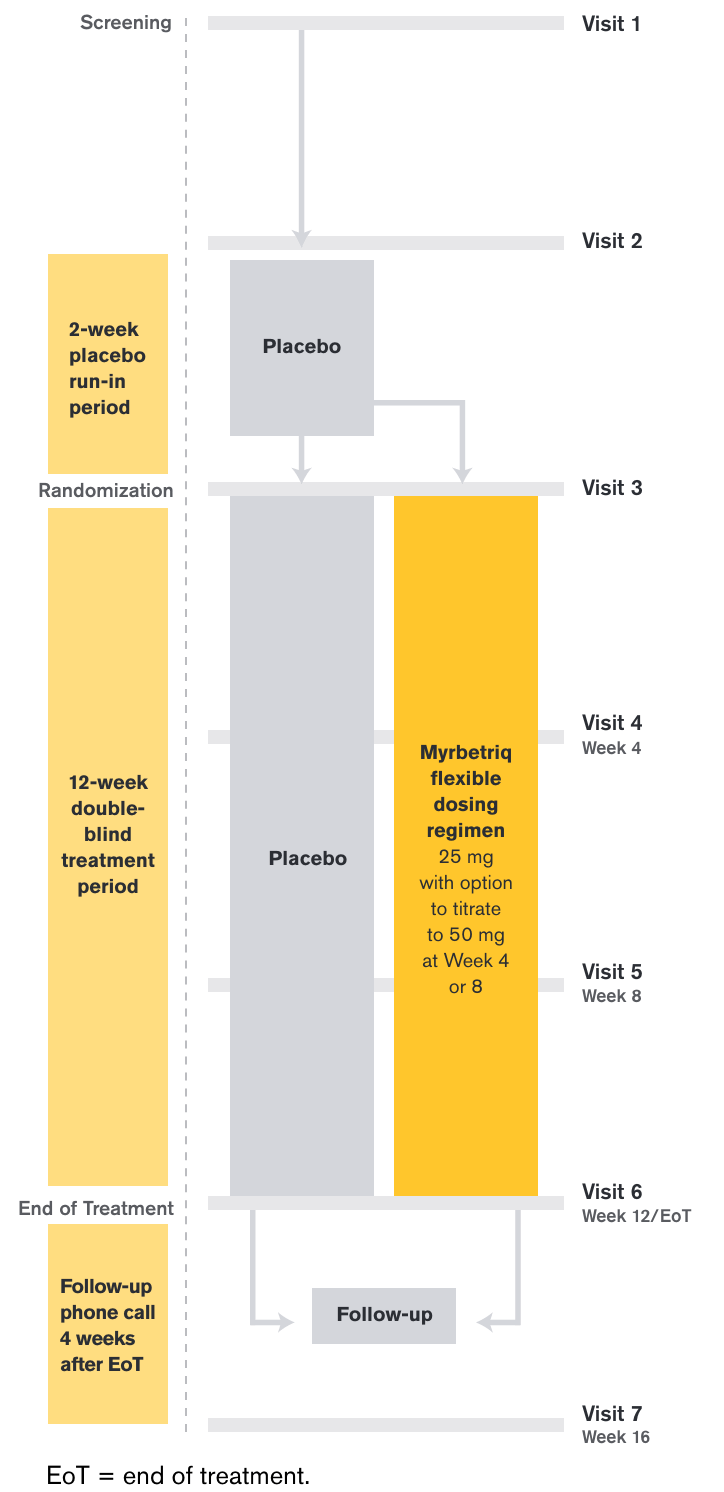
Treatment Arms and Timeline
After a 2-week placebo run-in period, those who entered the 12-week treatment period were randomized to Myrbetriq 25 mg or placebo and were given the option to increase to 50 mg at Week 4 or Week 8 based on individual efficacy, tolerability, and investigator discretion.
This study was designed to detect a difference between placebo and total mirabegron groups and not for each individual mirabegron dosing group.
PILLAR Efficacy Results
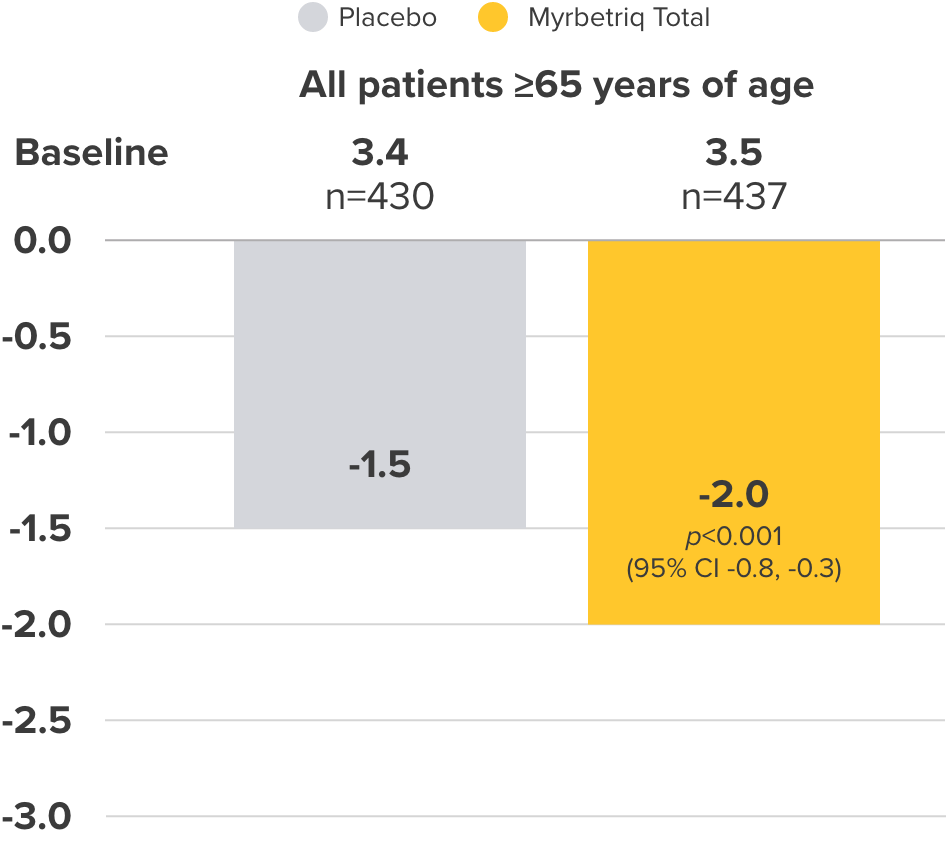
Adjusted mean change from Baseline to EoT3
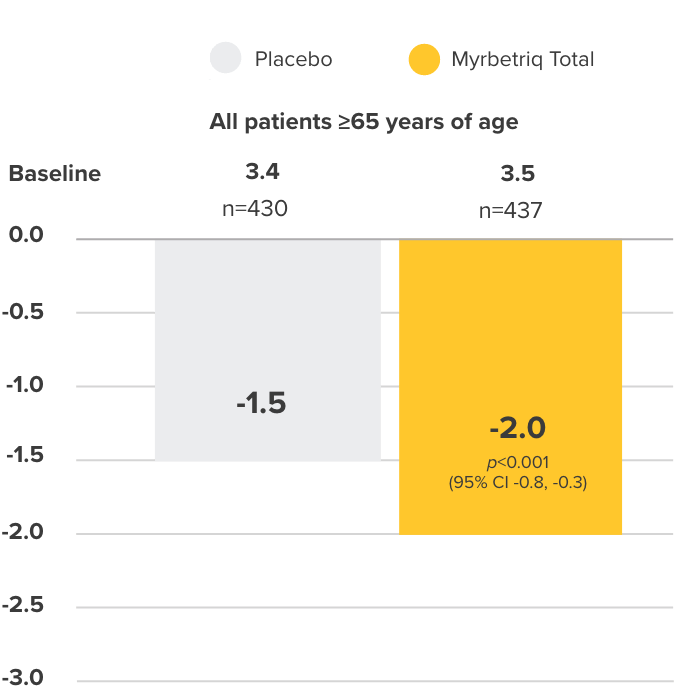
Co-primary Endpoint
Incontinence episodes
per 24 hours
Change from baseline to end of treatment (EoT) in mean number of urinary incontinence episodes per 24 hours3
- 38% of elderly patients receiving Myrbetriq achieved zero incontinence vs 30% in the placebo group (OR 1.50, 95% CI 1.09, 2.06)
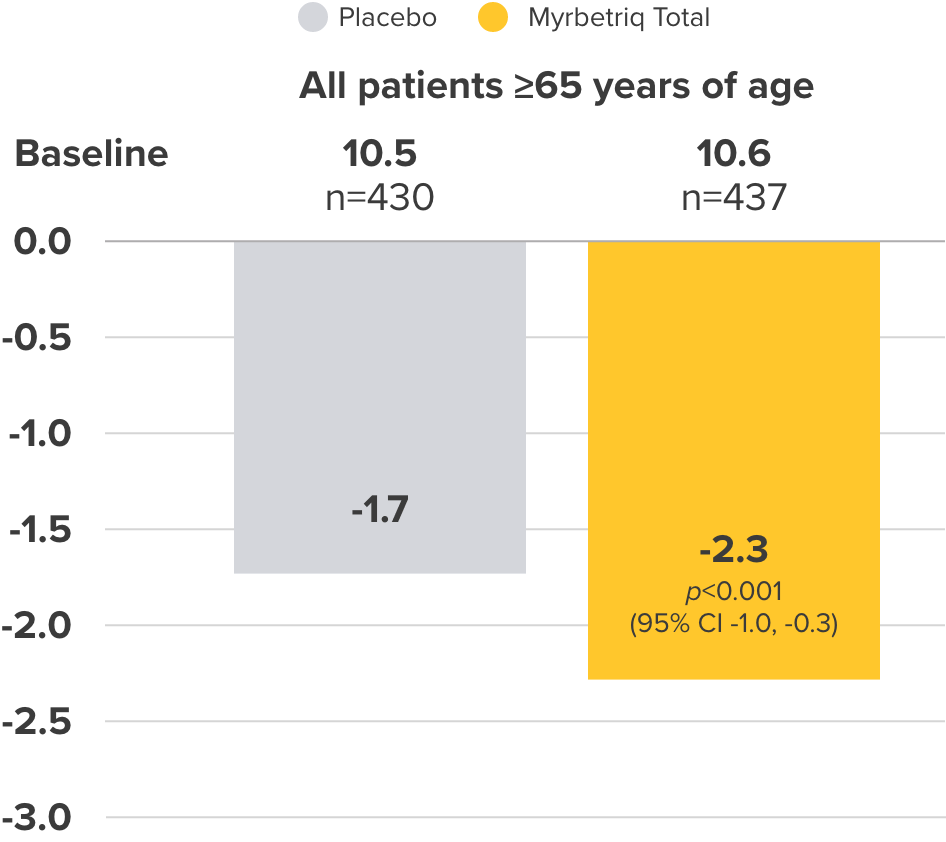
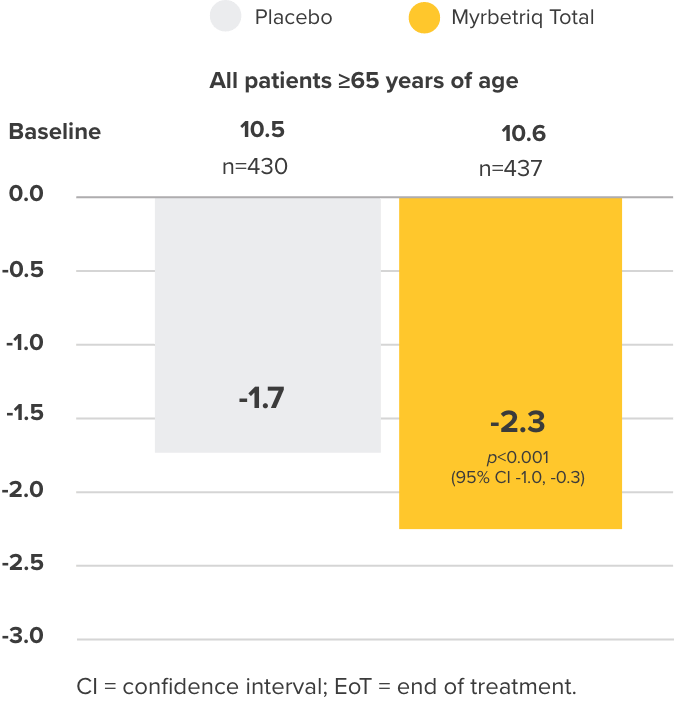
Co-primary Endpoint
Micturition frequency
per 24 hours
Change from baseline to EoT in the mean number of micturitions per 24 hours3
PILLAR Safety and Tolerability Results
Percent of patients with treatment-emergent adverse events (TEAEs)‡ who received ≥1 dose of study medication3
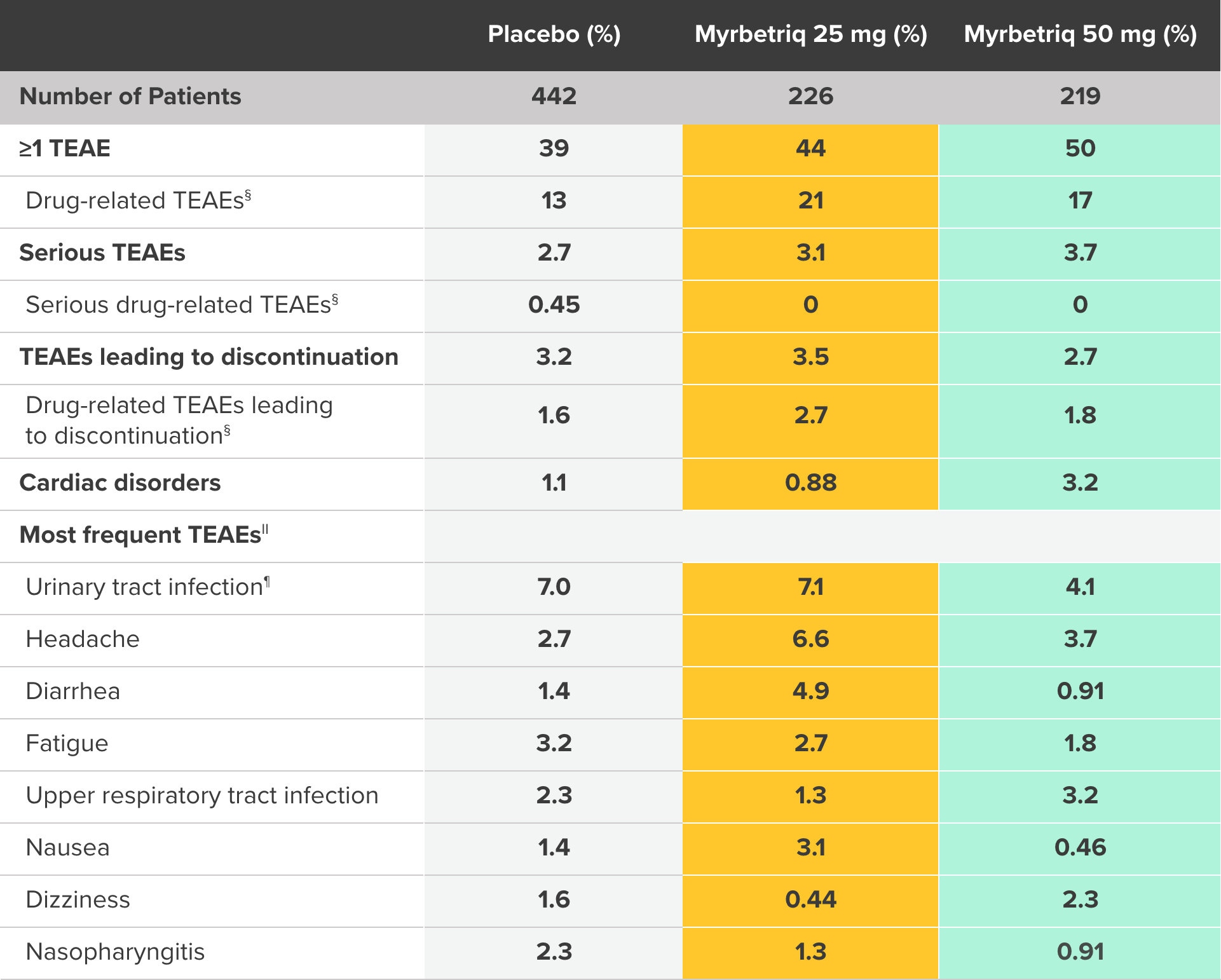
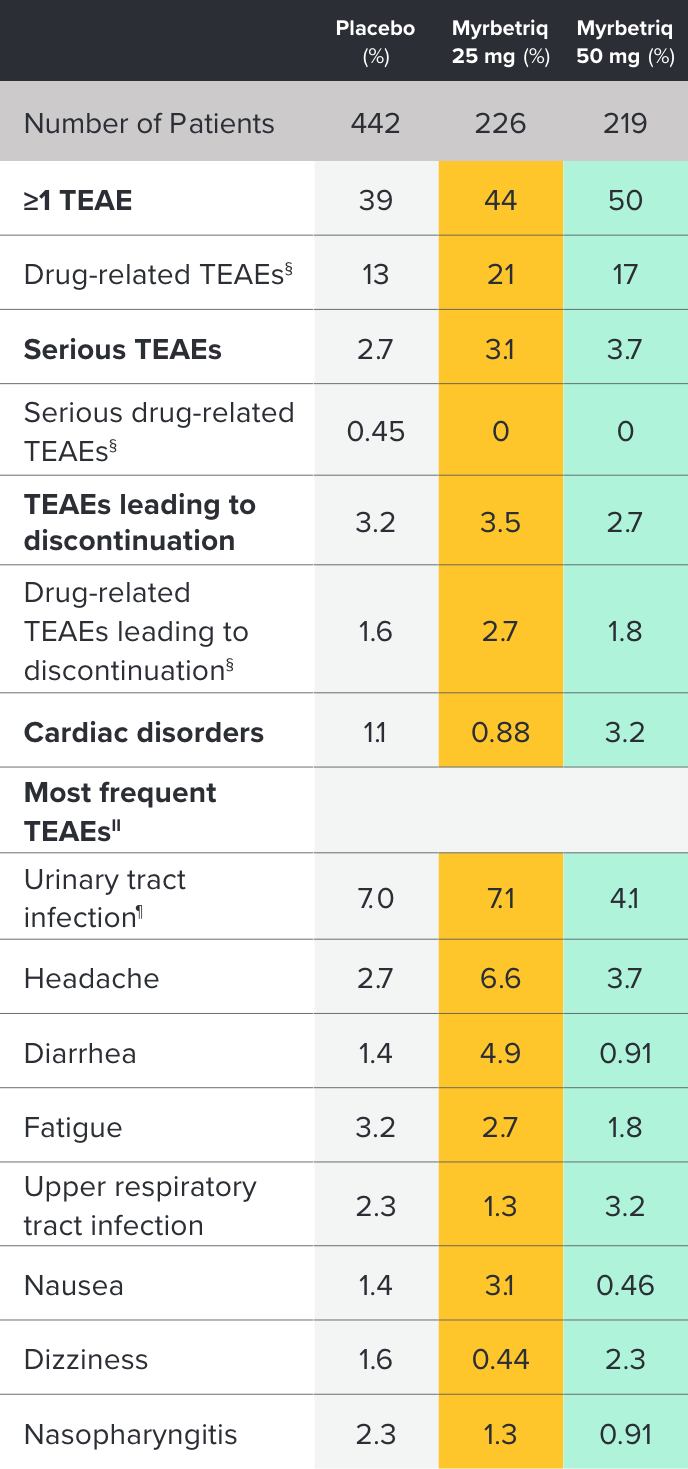
‡ Treatment-emergent adverse event (TEAE) is defined as an adverse event which started or worsened in the period from first double-blind medication intake until 30 days after the last double-blind medication intake.
§ Possible or probable, as assessed by the investigator, or where relationship was missing.
II Preferred term; affecting ≥2% of any treatment group.
¶ Escherichia urinary tract infection, streptococcal urinary tract infection, urinary tract infection, or bacterial urinary tract infection.
Myrbetriq is covered without restrictions for 99% of insured patients in Medicare Plans2**††
FORMULARY STATUS DOES NOT IMPLY SAFETY OR EFFICACY OF ANY PRODUCT; NO COMPARISONS SHOULD BE MADE.
** Provider communication only—not approved for prescription drug plan member distribution. Formulary status is not a guarantee. Please verify copay, coverage, and updated information with the plan sponsors. Information subject to change without notice. Astellas does not endorse any individual plan. †† By Medicare total covered lives (49,770,999); Plan types: Medicare MA, Medicare PDP, Source: Managed Markets Insight & Technology as of February 28, 2023. Without restrictions=no prior authorization, no step therapy, and no generic first.